OpenClinica
See the following -
Is The 1.5+ Trillion Dollar HITECH Act a Failure?
 Hopefully, the public statements made by President Obama and Vice President Biden will lead to a public debate over the monumental problems that the HITECH Act and proprietary EHR vendors have caused the American people. While the press continues to report the figure of $35 billion as the cost of implementing EHRs, that figure does not tell the entire story. Perhaps the next step is to provide accountability and transparency. That would start with firm numbers regarding the real costs of EHR implementations forced on an unprepared healthcare system by the HITECH Act.
Hopefully, the public statements made by President Obama and Vice President Biden will lead to a public debate over the monumental problems that the HITECH Act and proprietary EHR vendors have caused the American people. While the press continues to report the figure of $35 billion as the cost of implementing EHRs, that figure does not tell the entire story. Perhaps the next step is to provide accountability and transparency. That would start with firm numbers regarding the real costs of EHR implementations forced on an unprepared healthcare system by the HITECH Act.
- The Future Is Open
- Login to post comments
John Wilbanks to Keynote OpenClinica 2015 Conference (OC15)
 Noted advocate of open access in clinical research and Chief Commons Officer at Sage Bionetworks, John Wilbanks, will deliver the keynote presentation at the 2015 OpenClinica Global Conference (OC15), to be held May 31 - June 1 in Amsterdam. OpenClinica is the largest open source community in the clinical research field, and OC15 will bring together both clinical research and IT professionals to share cutting-edge information and ideas around how open source is being used to transform the clinical research landscape.
Noted advocate of open access in clinical research and Chief Commons Officer at Sage Bionetworks, John Wilbanks, will deliver the keynote presentation at the 2015 OpenClinica Global Conference (OC15), to be held May 31 - June 1 in Amsterdam. OpenClinica is the largest open source community in the clinical research field, and OC15 will bring together both clinical research and IT professionals to share cutting-edge information and ideas around how open source is being used to transform the clinical research landscape.
- Login to post comments
K&L Consulting Services, Inc. Of Fort Washington, PA Selects OpenClinica Enterprise Clinical Trial Software
K&L looks to leading commercial open source player to support its growing global CRO practice. Read More »
- Login to post comments
Latest Release of OpenClinica now available
We are thrilled to announce the general availability OpenClinica 3.1.3. This latest release of the OpenClinica clinical trial software provides over 100 fixes and enhancements, including: Read More »
- Login to post comments
Leader in Clinical Research Tech Reacts to Apple's #ResearchKit
 Apple, Inc. has a remarkable ability to capture the world’s attention when announcing “the next big thing.” They have honed their well-known Reality Distortion Field skills for over 30 years...ResearchKit has grabbed such attention. Maybe not as much as The Watch, but amongst the minority of us who pay attention to such things. And the reactions have been typically polarized—it’s either an “ethics quagmire” or “Apple fixing the world.” But reality rarely presents an either-or proposition...
Apple, Inc. has a remarkable ability to capture the world’s attention when announcing “the next big thing.” They have honed their well-known Reality Distortion Field skills for over 30 years...ResearchKit has grabbed such attention. Maybe not as much as The Watch, but amongst the minority of us who pay attention to such things. And the reactions have been typically polarized—it’s either an “ethics quagmire” or “Apple fixing the world.” But reality rarely presents an either-or proposition...
- Login to post comments
Obama and Biden Blast EHR Vendors for Data Blocking
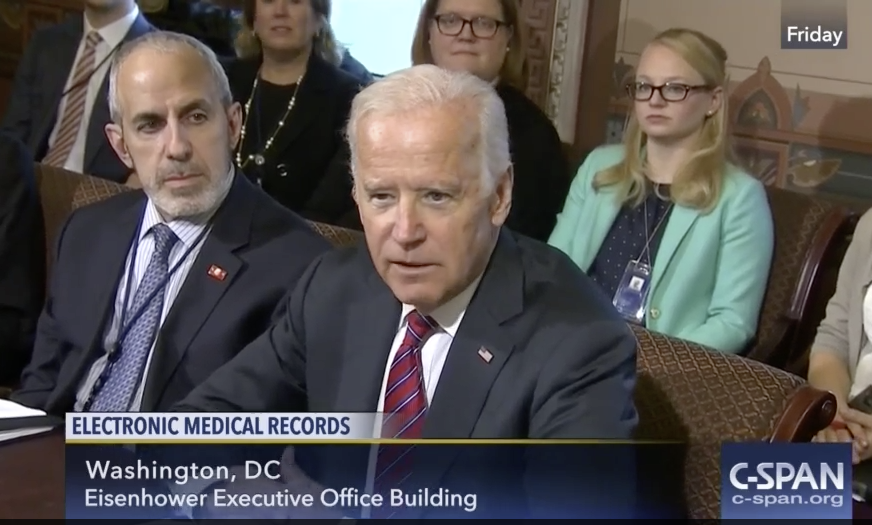 As they are winding their terms in office, President Barack Obama and Vice President Joe Biden dropped a stink bomb on the health IT industry. Speaking at different events on Friday, January 9th, the President and Vice President both criticized proprietary electronic health record (EHR) vendors as the primary obstacle to the success of their administration’s health care strategy. This is the highest level acknowledgment so far of the serious impact that “lock-in” EHR software vendors are having on America’s medical infrastructure and the ability of physicians to provide medical care.
As they are winding their terms in office, President Barack Obama and Vice President Joe Biden dropped a stink bomb on the health IT industry. Speaking at different events on Friday, January 9th, the President and Vice President both criticized proprietary electronic health record (EHR) vendors as the primary obstacle to the success of their administration’s health care strategy. This is the highest level acknowledgment so far of the serious impact that “lock-in” EHR software vendors are having on America’s medical infrastructure and the ability of physicians to provide medical care.
Open Source Clinical Software is Opening Up Biotech
Open-source computing looks at software as a public good. Programs are measured in pride over profit. Programs are provided with their source code free-of-charge, allowing users to freely modify the work, provided the terms of the license (usually attribution and licensing the derived code under the same open-source license) are met. Read More »
- Login to post comments
Open Source Software Creeps into Healthcare through Clinical Research
 Although open source has not conquered the lucrative market for electronic health records (EHRs) used by hospital systems and increasingly by doctors, it is making strides in many other important areas of health care. One example is clinical research, as evidenced by OpenClinica in field of Electronic Data Capture (EDC) and LabKey for data integration. Last week I attended a conference for people who use OpenClinica in their research or want to make their software work with it. At any one time, hundreds of thousands of clinical trials are going on around the world, many listed on an FDA site. Many are low-budget and would be reduced to using Excel spreadsheets to store data if they didn’t have the Community edition of OpenClinica. Read More »
Although open source has not conquered the lucrative market for electronic health records (EHRs) used by hospital systems and increasingly by doctors, it is making strides in many other important areas of health care. One example is clinical research, as evidenced by OpenClinica in field of Electronic Data Capture (EDC) and LabKey for data integration. Last week I attended a conference for people who use OpenClinica in their research or want to make their software work with it. At any one time, hundreds of thousands of clinical trials are going on around the world, many listed on an FDA site. Many are low-budget and would be reduced to using Excel spreadsheets to store data if they didn’t have the Community edition of OpenClinica. Read More »
OpenClinica
OpenClinica is a leading open source clinical trials software for electronic data capture (EDC) and clinical data management (CDM). OpenClinica allows researchers to build studies, design electronic Case Report Forms (eCRFs), and conduct a range of clinical data capture and clinical data management functions. Read More »
- Login to post comments
OpenClinica And TraIT: A Dutch National Research Infrastructure
Is it possible to set up an IT infrastructure for translational research for an entire country? The Dutch Translational Research IT (TraIT) project (http://www.ctmm-trait.nl/) believes it is. Admittedly, The Netherlands is not exactly the same size as China or the US, but nevertheless already 26 partners from industry and academia to collaborate in this consortium to organize, deploy, and manage a nationwide IT infrastructure for data and workflow management targeted specifically at the needs of translational research.
- Login to post comments
OpenClinica Announces New Software Release; Leverages Community Development Model For EDC
OpenClinica, LLC announces the latest version of the most prolific open source electronic data capture platform. The new version (3.2) includes, among other items, performance enhancements, internationalization enhancements, a stack upgrade, and printable subject casebooks with full provenance data.
- Login to post comments
OpenClinica At Netherlands CTMM Annual Event
On September 12, 2013, The Center for Translational Molecular Medicine (CTMM) held their annual meeting at the Media Plaza, Jaarbeurs Utrecht in the Netherlands. The theme, ‘Let’s talk about Value,’ celebrated the implementation of proven results in a clinical practice, the benefits they will bring for patients, and the real value they will add to society and the Dutch economy.
- Login to post comments
OpenClinica chosen for use in Cardiovascular Clinical Trials
Global producer of cardiovascular therapies looks to commercial open source software to support key clinical research.
OpenClinica, LLC announces that TriReme Medical, a global producer of therapies for complex vascular disease, has selected the OpenClinica Enterprise Edition to enhance data collection and management in its clinical research. Read More »
- Login to post comments
OpenClinica Enterprise Works For Boutique CROs Like Cancer InCITe
OpenClinica, LLC announces that Cancer InCITe, LLC of San Antonio, Texas has selected the OpenClinica Enterprise Edition for Electronic Data Capture (EDC) and clinical data management for phase 1 and phase 2 clinical trials. [...] Read More »
- Login to post comments
OpenClinica Enterprise Works for Boutique CROs Like Cancer InCITe
OpenClinica, LLC announces that Cancer inCITe, LLC of San Antonio, Texas has selected the OpenClinica Enterprise Edition for Electronic Data Capture (EDC) and clinical data management for phase 1 and phase 2 clinical trials.
- Login to post comments